Sacrificial Anodes FAQs
Q. What do sacrificial anodes do?
A. All metals immersed in an electrolyte (sea water for example) produce an electrical voltage. When two dissimilar metals are in contact (electrically connected) they produce a galvanic cell (like a battery), with the less noble metal (a bronze propeller for example) forming the anode and the more noble metal (stainless steel shaft) forming the cathode.
Aluminum anode alloy provides more protection and lasts longer than zinc. It will continue to work in freshwater and is safe for use in salt water. Aluminum is the only anode that is safe for all applications.
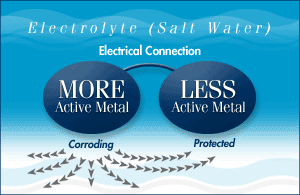
If you want to protect both metals you need to connect a third metal that is more active than the first two. The most active metal (zinc for example) becomes the anode to the others and sacrifices itself by corroding (giving up metal) to protect the cathode - hence the term sacrificial anode.
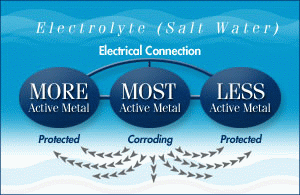
Q. What metals are sacrificial anodes made from?
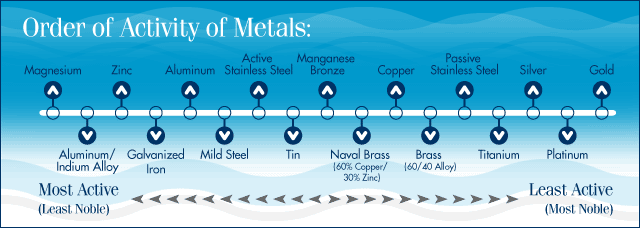
A. The three most active materials used in sacrificial anodes are zinc, aluminum and magnesium. They have different properties and uses.
The first property to consider is their electrical potential. All metals generate a negative voltage (as compared to a reference electrode) when immersed in water. The lower – the more negative - the voltage, the more active the metal is considered to be, for example:
Magnesium generates -1.6 Volts, i.e. negative 1.6 volts.
Aluminum sacrificial anode alloy generates -1.1Volts
Zinc, -1.05 Volts
In order to provide protection, the highest practicable voltage difference possible is required between the sacrificial anode and the metal to be protected.
For example, if zinc is used to protect a bronze propeller, a “driving or protecting voltage” of negative –0.75 volts will be available, i.e. zinc at -1.05 volts minus bronze at -0.3 = - 0.75 volts.
If aluminum anodes are used this increases to -0.8 Volts.
Magnesium anodes increase this to -1.3 volts.
The bigger the difference in voltage, the more protection you get. But, beware, some materials (aluminum) can be “overprotected” – more about that later.
The second property that is important is the current capacity of the anode material. The anode generates a voltage difference and this drives a current between the anode and the protected metal and through the water. It’s like having a bigger battery, the more capacity you have the longer it will keep protecting. Incidentally, for a particular anode, the rate of current flow is dependant on the surface area of the anode and the longevity depends on the mass. For the same size anode the relative capacities are:
Zinc: 100 (Taken as a datum e.g. this could be 100 days)
Magnesium: 30
Aluminum anode: 130 – 150 (Different manufacturers quote different ranges)
So if you used a magnesium anode in place of the “100 day” zinc anode it would only last 30 days.
The aluminum anode would last between 130 and 150 days.
For convenience we provide the "Zinc Equivalent Weight" (ZEW) in some of our product listing pages. This is the amount of zinc that the aluminum anode is equivalent to in terms of capacity.
The third property is Quality of the Anode Alloy
A word of caution about the metals used !!
Not just any zinc or any aluminum will work. Beware ! There are some imported anodes which are of questionable quality. It is important to ensure that the anodes you buy are made to the appropriate military or marine specification. Installing cheap or sub-standard anodes will undoubtedly cause increased and potentially very expensive corrosion problems. The most common material specifications are:
Zinc: MIL-DTL-18001L
Aluminum: MIL-DTL-24779(SH)
Magnesium: MIL-A-21412
Comparison of Properties

Q. What anode material should I use on my boat?
A. The type of boat that you have will determine how careful you need to be. A fiberglass hull with an inboard engine will need much less protection than an aluminum hull or a boat with an aluminum sterndrive for example. Some simple guidelines:
Inboard boats with mainly bronze and stainless metal parts can be protected using zinc or aluminum anodes. Don’t worry about overprotecting them. You are only overprotected when the weight of the anodes is so great that your boat sinks! The voltage generated by zinc or aluminum anodes will not cause any damage – no matter how much anode material is added, the maximum voltage that can be generated is the voltage of the anode itself. You could also use magnesium in freshwater locations on fiberglass-hulled boats. Be careful using magnesium on aluminum or wooden hulled boats since you can overprotect them. Steel hulls can also be overprotected to the point where excessive protection voltage rapidly lifts the paint off the hull.
Sterndrives and Outboard Motors require a little more care. The sacrificial anodes have a difficult task, since they have to protect what is already a very active aluminum assembly. Initially the anodes for these units were made of zinc, but in response to corrosion problems, Mercury and Johnson/Evinrude/OMC started selling the aluminum anodes in the early 1990’s. Other manufacturers are switching to aluminum too. The small increase in protective voltage helps ensure that the sterndrive is protected. If you use zinc anodes you may even invalidate your warranty! Again, be careful using magnesium anodes since you can overprotect your sterndrive or outboard.
Q. What anodes should I use in freshwater?
A. Where possible Navalloy™ (aluminum/zinc/indium alloy) anodes are recommended over zinc. Zinc anodes can become inactive after only a few months due to the build up of an insulating film of zinc hydroxide. Aluminum anodes will remain active. Don't take our word for it though, ABYC (American Boat and Yacht Council), who set the standards for the industry, clarified their recommendations on anode materials in the Standards and Technical Information Reports for Small Craft (July 2008-2009):
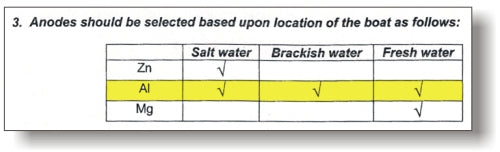
As you can see, the only anode type that is recommended for all water types is aluminum (Navalloy).
This chart summarizes the anode choices based on type of boat and water type:

Q. What factors increase corrosion?
A. The voltage difference between the two metals will affect the rate of corrosion. For example a stainless steel prop, which is a relatively noble metal, will cause more corrosion of a set of zincs than a bronze prop. Corrosion will increase the saltier the water is. Increasing temperature will also increase the conductivity of water and the resulting corrosion. The corrosion rate doubles with every 10 degrees Celsius (18 degrees Fahrenheit) increase in temperature. Pollution can also increase corrosion. For example, many freshwater lakes have been contaminated by acid rain, which increases the conductivity of the water and therefore corrosion rates.
Q. When should sacrificial anodes be replaced?
A. Anodes should be changed, at least, on an annual basis (including anodes in fresh water) or when they have corroded to half their original size. Performance Metals “Premium” anodes include the exclusive patented “wear indicator.” When the red spot appears it is time to change your anode!
Q. What precautions should I take when installing new anodes?
A. Make sure they make good electrical contact with the metal that is being protected. Remove any paint and clean the metal surface that will be in contact with the anode. DON’T paint anodes! They can’t work if they are covered up.
Q. What else should I do to help protect my sterndrive?
A. Keep paint (on engines, sterndrive units etc.) in good condition. A small scratch will corrode rapidly. Leave the sterndrive unit immersed in the water. If you don’t the anodes can’t work. Don’t use anti-fouling paint containing copper or mercury on a sterndrive unit. The metal in the paint will increase galvanic corrosion. Don’t mix zinc anodes on the hull with aluminum anodes on the drive. The aluminum anodes will protect the zinc anodes in addition to the unit.
Q. How can Navalloy™ (aluminum) anodes protect aluminum outdrives?
A. Because the Navalloy™, aluminum anode is a combination of aluminum, zinc and indium. It is like comparing steel and stainless steel - they have very different properties. The zinc and indium make the metal more active and prevent the anode from forming an oxide coating.
Useful Documents
Bonding and Cathodic Protection of Vessels - by Paul Fleury of Marine Services pdf
The Truth About Aluminum Anodes - by Martin Wigg and Paul Fleury pdf
ABYC Recommendations on Anode Materials- Advertising material pdf
Strap Anode Flyer - Advertising material pdf
SecureCore® Flyer - Advertising material pdf
PowerPoint Basic Corrosion Course - Explains the basics of Galvanic corrosion and how sacrificial anodes work. pdf
Corrosion Course - pdf version
